Advanced Right Heart Disease
This chapter discusses echocardiographic subtleties of unusual right heart diseases.
A detailed examination of the right ventricle (RV), right atrium (RA), tricuspid valve (TV), pulmonic valve (PV), and pulmonary artery (PA) is required to diagnose some essential diseases. Early diagnosis of some of these diseases, when symptoms are subtle or absent, produces the best patient outcomes.
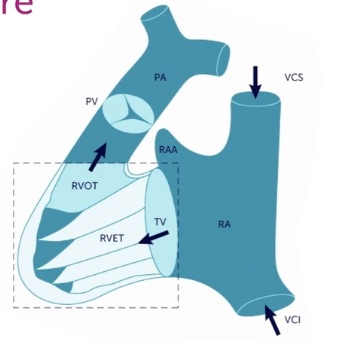
Here is a schematic image showing the complicated shape of the RV, which early anatomists compared to a bagpipe. The RV INFLOW region and apex are the bag, and the RV OUTFLOW is the pipe going to the PV and PA.
No single view can image both the RV INFLOW and RV OUTFLOW regions. The Heart Views, RV Size/Function, and Tricuspid Valve chapters discussed details about the RV INFLOW views. This chapter focuses on the RV OUTFLOW views needed when a patient has an elevated TV gradient or a dilated RV. In an RV Outflow view, we place Color over the PV and obtain a PW tracing in the RVOT just before the PV. When pulmonic stenosis (PS) is possible, we also put CW through the distal RVOT and PV.
If all heart windows had good views, we would choose the subcostal window for the RV Outflow view because it is most parallel to the flow. However, the subcostal window may be suboptimal, and we then need options in the parasternal and apical windows.
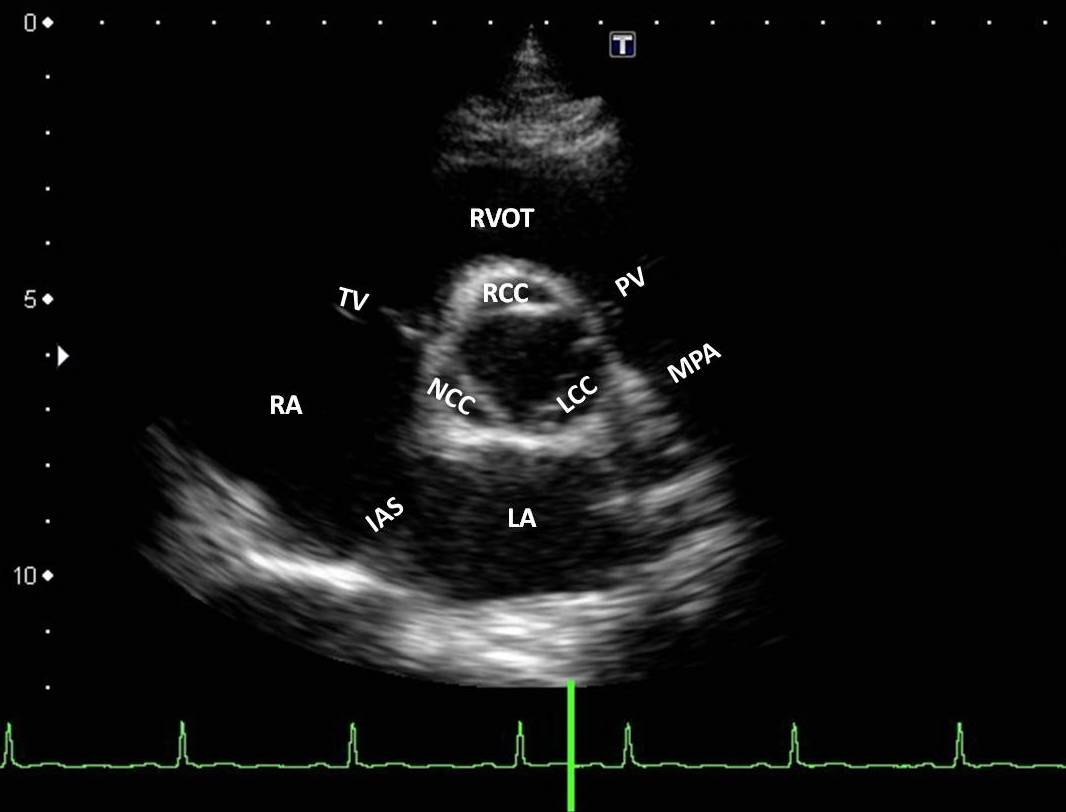
PSAX: If the familiar PSAX is fanned cephalad, we can view the distal RVOT and may be able to angle to the patient’s left to see the PV. Here is a labeled PSAX image from the Austin Community College echocardiography program using a patient with a small heart.Notice that the RVOT curves anterior to the ascending aorta and then moves posteriorly. The PV is a little anterior and to the patient’s left of the AV. This view is not parallel to flow through the RV Outflow, but Color and PW before the PV can still give useful information.
APICAL5+: This view is obtained by fanning more anterior than the apical5. On the screen, the RVOT appears anterior to the AV, running from 10:00 to 4:00. This view is more parallel to flow than the PSAX but not as parallel as the subcostal views. Here is an acceptable view:
SC5+: This is an excellent view with a good subcostal window. Obtain the SC4 and fan anteriorly past the subcostal5 to see a vertical RVOT and PV parallel to the flow.
SCSAX RV OUT: Rotate 90 degrees counterclockwise from the SC4 and find the RV Outflow to the right of the AV on the screen. This view has an anterior/posterior plane through the RVOT and may be better than the SC5+ in some patients. Here is a normal SCSAX with the tricuspid valve at about 12:00 and the PV at about 3:00.
RIGHT VENTRICULAR DILATION
This is a crucial finding in right heart disease. Eyeballing may quickly identify generalized RV dilation, but some right heart diseases are distal and focal, so measurement at the following four locations may be needed (normal ranges shown).
RVEDD base: 2.5 - 4.1 cm
RVEDD mid: 1.9 - 3.5 cm
RVOT proximal: 2.1 - 3.5 cm
RVOT distal: 1.7 - 2.7 cm
Body height must be considered when interpreting a patient’s measurements. There isn’t a routinely published range for the RV apex diameter, but it should be smaller than the mid-RVEDD. The length of the RV was measured in the past but doesn’t add much, so we don’t do it. Here are the diagnostic considerations for RV dilation.
- Pressure Overload
- Pulmonary arterial hypertension (PAH)
- Pulmonary embolism (acute or chronic) (PE)
- Pulmonic stenosis (PS)
- Volume Overload
- Tricuspid regurgitation (TR)
- Pulmonic regurgitation (PR)
- Left to right shunts (ASD, VSD, PDA, coronary fistulas)
- RV wall abnormalities
- RV infarct
- RV dysplasia
PULMONARY ARTERIAL HYPERTENSION (PAH)
The RV does not compensate sufficiently for elevated afterload, so untreated patients with substantial PAH have short survival. Idiopathic PAH is optimally diagnosed in asymptomatic patients who can be treated “early and hard” with multiple drugs before advanced RV dilation and dysfunction have occurred.
Measuring PA systolic pressure directly, but non-invasively, is difficult, but the peak systolic TV gradient indirectly reflects this pressure. Remember that severe PAH does not always produce moderate or severe TR, so even trace TR should have the gradient measured. Chronic, substantial PAH eventually causes diffuse RV hypertrophy and dilation, which will worsen TR. In the later stages of PAH, increased systolic IVS flattening/D-ing toward the LV develops, and cardiac output further deteriorates. Because mild or moderate pericardial effusions are frequent in advanced PAH, pericardial tamponade is sometimes considered. However, the prominent RV hypertrophy and dilation with a high TV gradient should strongly favor PAH. When the RA pressure exceeds LA pressure, hypoxia can develop if blood can shunt right to left through a patent foramen ovale.
The catheterization lab cutoff for diagnosing PAH was lowered to a mean pulmonary artery pressure > 20 mmHg because of significant improvements in early PAH treatment. An IMBUS physician should be concerned about PAH when the peak systolic TV gradient exceeds 27 mmHg, particularly in non-elderly patients. Peak gradients between 22-27 mmHg are still abnormal but can be followed periodically to watch for a rise in the gradient.
Remember to exclude the rare condition of pulmonic stenosis (PS) with Color and Doppler analysis in any patient with an elevated TV gradient (see the Carcinoid section at the end of this chapter). Patients with high tricuspid valve gradients should undergo the following diagnostic process after PS is excluded.
-------------
STEP 1: The first diagnostic step for an elevated TV gradient is to search for post-capillary disease, much of which is done during our exam of the left heart.
Post-capillary PAH
Post-capillary PAH is caused by some disease of the left heart that chronically elevates the left atrial, then the pulmonary venous, and finally, the pulmonary arterial pressures. In the traditional 5-group classification of PAH, this is Group 2. The diseases may be LV systolic dysfunction, LV diastolic dysfunction, Valve disease (mitral more common than aortic), Arrhythmias, and infrequently Pacemaker troubles. Fixing the left heart can reduce the PAH. The left atrium would have to be dilated in these conditions before PAH would be induced.
In the section on Acute PE below, systolic notching of the distal RVOT PW tracing is described in more detail as a marker of hemodynamically significant PE. However, this finding more generally indicates pre-capillary pulmonary hypertension, inconsistent with left heart disease. Therefore, PW before the PV should be done in any patient with an elevated TV gradient using the best available RV OUTFLOW view. Since the finding is a visual pattern and not a measured velocity, a less-than-parallel view can still work, but the subcostal views should be best.
The definitive test in equivocal cases is the pulmonary artery wedge pressure during right heart catheterization. A resting wedge pressure substantially < 15 mmHg excludes post-capillary PAH.
Pre-capillary PAH
Pre-capillary PAH is the working diagnosis when an elevated TV gradient is not caused by PS or left heart disease. The following differential diagnosis needs to be considered.
Group 1: Idiopathic PAH (the disease with significant, new treatments), Heritable (rare), Drugs/toxins (mostly amphetamines and anorexants), Connective tissue diseases (e.g., systemic sclerosis), HIV, Portal hypertension, Schistosomiasis, and Pulmonary veno-occlusive disease (rare and should have a diffuse interstitial pattern with pulmonary IMBUS).
Group 3: COPD seldom causes substantial PAH. The rarer interstitial pulmonary diseases can be a cause and should produce a diffuse interstitial pattern with pulmonary IMBUS. Severe sleep-disordered breathing with sustained hypoxia may need to be considered in some patients, and many cardiologists routinely want an assessment for sleep hypoxia.
Group 4: Chronic thromboembolic pulmonary hypertension (CTEPH) is a significant group that is more common than either group 1 or 3 and has specific, effective interventional treatment. This is discussed in more detail below.
Group 5: A tiny, multifactorial grab bag for poorly understood patients.
-------------
STEP 2 WHEN PRE-CAPILLARY PAH IS THE WORKING DIAGNOSIS: A patient’s history, pulmonary IMBUS exam, and sleep hypoxia evaluation will eliminate most patients in Groups 3 and 1 except for idiopathic PAH.
The primary task before diagnosing idiopathic PAH is excluding group 4, CTEPH. Several different types of imaging can help with this determination. A direct discussion with radiology may be needed to choose the correct exam, but cardiology will usually be involved. Occasionally, invasive pulmonary angiography is necessary.
Acute PE:
Before discussing CTEPH, here is a quick review of echocardiography in acute PE. A PE needs to acutely obstruct at least 20-30% of the pulmonary vascular bed to cause hemodynamic compromise, so most patients with PE have normal right heart echocardiography. When an acute PE is large enough, the afterload on the RV increases, and a previously normal RV quickly dilates and declines in function. TR can result, and the peak gradient may be elevated but not > 60 mmHg because a non-hypertrophied RV cannot acutely generate higher pressures no matter how severe the obstruction. Systolic flattening/D-ing of the IVS can occur. The apical RV function can be more preserved than the mid- and basal RV function, called a McConnell sign. While this sign is not very sensitive or completely specific, it can still be an essential clue. Here is an example of a McConnell sign showing the apex winking in.
The following image is from one of our hospital patients with a massive PE. Although the image quality is worse, a fuller view of the RV makes it easier to see the discrepancy between the radial function at the base and mid-RV compared to the apex.
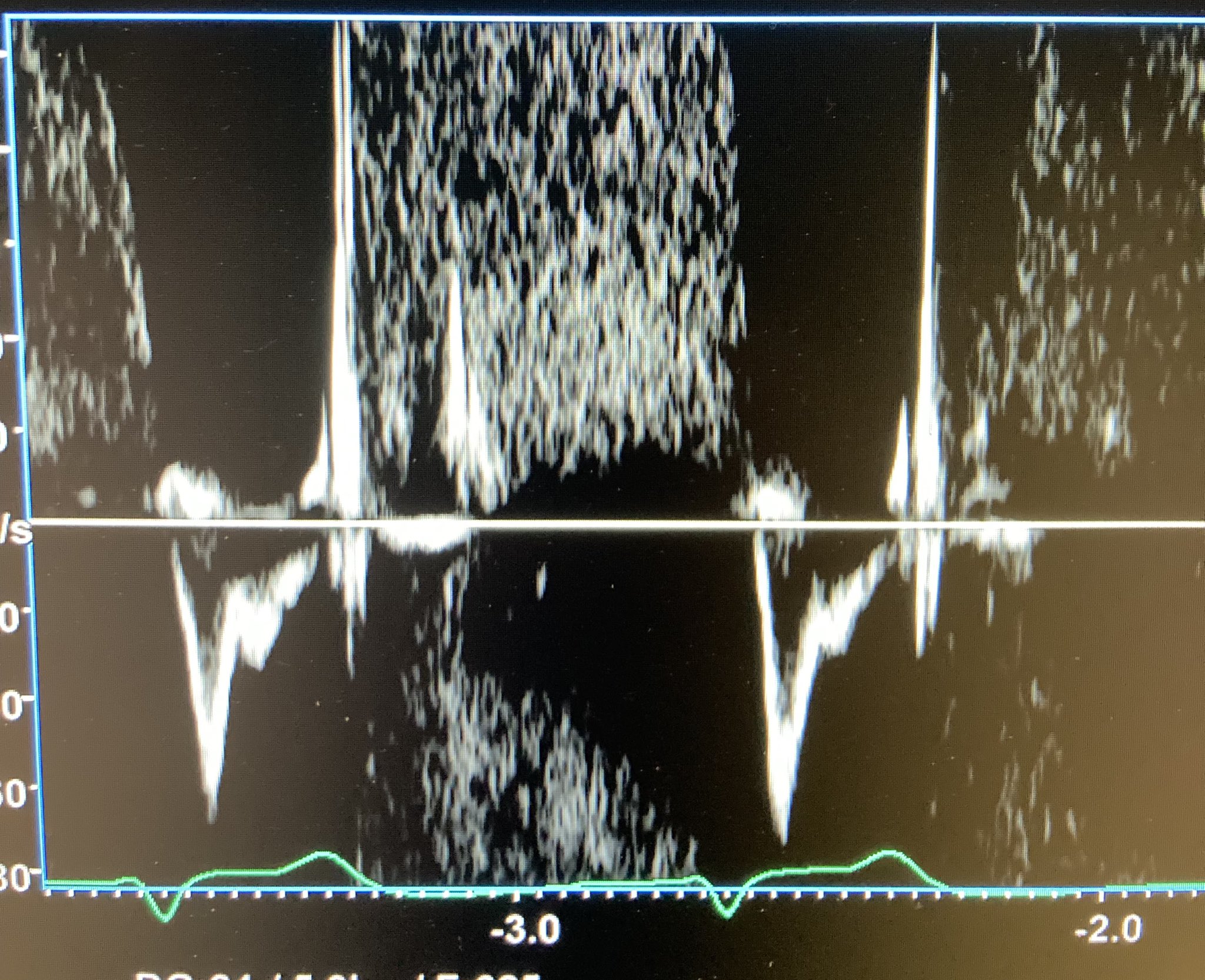
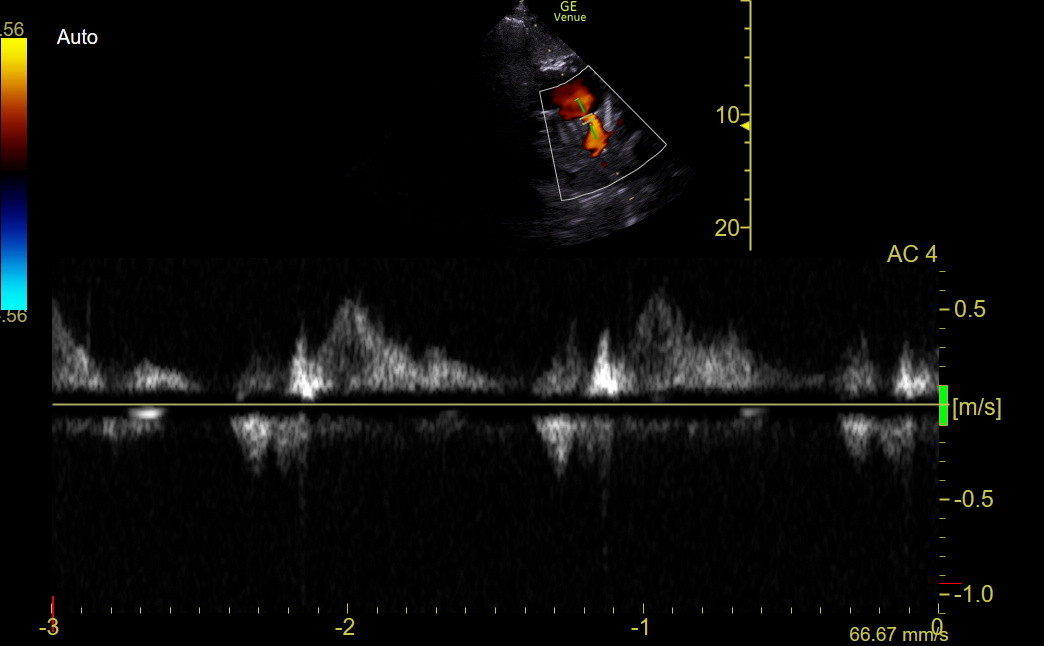
However, the best marker of hemodynamically important PE is “systolic notching” of the distal RVOT PW tracing, indicating substantial pre-capillary pulmonary hypertension (Afonso, JASE 2019;32:799-806). Use the best RV outflow view and put the PW gate before the PV. In a large study, systolic notching was 92% sensitive and 99% specific, with high interobserver agreement for ≥ submassive PE. It was more sensitive than McConnell’s sign or other hemodynamic measurements.
However, as noted above, systolic notching indicates ≥ moderate precapillary PAH, regardless of cause. A distinction is made between acute PAH and chronic PAH based on how early the notch appears in the tracing, but this isn’t an easy judgment. We think any notching of the distal RVOT PW signal markedly increases the chance of major PE in a patient unless other markers of chronic PAH are present. We don’t have an example of the notching in a PE patient, so here is an image from a Twitter-published case. Look in the bottom half of the figure for the notched distal RVOT PW tracing.
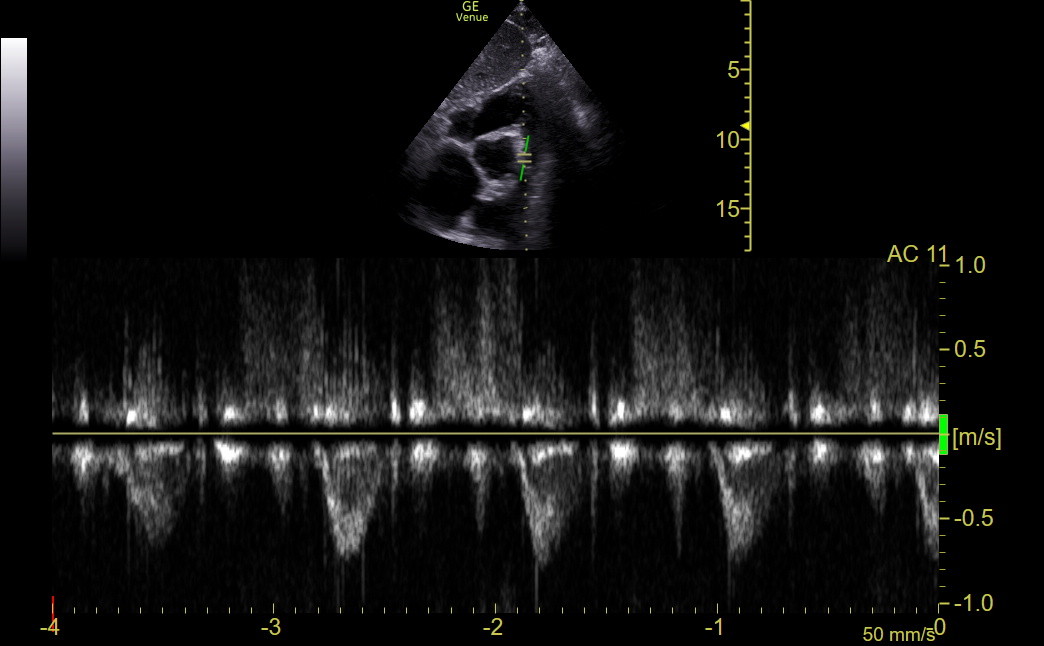
The following normal tracing was obtained with Venue using the SCSAX view for comparison.
We did have a clinic patient who, amongst other findings, had an elevated TV gradient and prominent PR in the SCSAX. The distal RVOT PW tracing shows the diastolic PR (above the line) and a mid-cycle notch in the distal systolic RVOT flow below the line, indicating substantial pre-capillary pulmonary hypertension.
When precapillary PAH is most severe, the RVOT tracing can show a sharp, brief, triangular pattern without a notch. This pattern is easily distinguished from a typical flow curve.
CTEPH:
CTEPH is a late complication of acute PE in a small percentage of patients. The echocardiographic appearance of CTEPH and idiopathic PAH is the same. The unclear mechanism may include recurrent PE and genetic susceptibility to poor clot lysis or over-aggressive vessel wall remodeling. Since CTEPH is sometimes diagnosed in patients without a previous diagnosis of PE, the assumption is that some patients with acute PE don’t seek medical attention or are misdiagnosed. Our clinic patients with recent PEs should have IMBUS screens for PAH during the first several years because CTEPH needs to be diagnosed before RV dilation and dysfunction have developed.
Balloon angioplasty and surgical thromboendarterectomy can dramatically help and even cure patients with CTEPH. Hence, they need to be cared for in a center that regularly performs these procedures. CTEPH patients are the only PAH patients who should be anticoagulated.
Idiopathic PAH:
The treatment of this disease is pharmacologic and complicated but has markedly improved the function and survival of these patients. Current medications can attack at least three mechanisms, and drugs for other targets are being developed. Experts favor “early and hard” treatment with several medications, even in minimally symptomatic patients.
RIGHT HEART VOLUME OVERLOAD
The RV can compensate for volume overload for many years. It dilates but doesn’t hypertrophy. However, it may eventually become irreversibly dysfunctional, with a poor prognosis. The significant lesions causing RV volume overload are TR, PR, and left-to-right shunts.
The RV in these patients has good function until late in the disease and may even appear hyperdynamic. Septal flattening or D-ing of the IVS in diastole is characteristic later in the disease. The internal jugular, IVC, and portal veins will reflect the elevated RA pressure.
Tricuspid Regurgitation (TR)
Tricuspid regurgitation is common, and many normal adults have trace TR, which can be ignored. However, more substantial TR must be followed carefully because the disease can be progressive, regardless of the cause. The RV volume overload from the TR causes increasing RV and RA dilation, worsening the TR in a vicious cycle.
Functional TR, which should have a central jet, occurs when the RV, or the RA, dilates. For example, chronic atrial fibrillation causes biatrial dilation, and this dilation alone can result in functional TR or MR. With RA dilation, or basal RV dilation, the TV annulus is stretched, moving the leaflets apart and producing a coaptation defect. If the RV dilation involves the mid-RV, the papillary muscles are displaced, pushing the chordae and further worsening coaptation. Transvenous devices can now repair functional TR, so patients with ≥ moderate TR need to be followed regularly so intervention can occur before irreversible damage has happened to the RV.
Structural TR can be from Rheumatic heart disease, Blunt chest trauma, Pacemaker leads (shown in the TV chapter), Endocarditis, Carcinoid heart disease, and Congenital conditions (including Ebstein anomaly).
Here is a patient with blunt chest trauma and severe TR. The PLAXR was used to show the flail anterior TV leaflet.
Ebstein anomaly is a congenital abnormality in which the TV is displaced apically in the RV. TR can be substantial; the RV is smaller, and the RA is larger because it contains an “atrialized” portion of the RV. Here is an Ebstein anomaly.
Pulmonic Regurgitation (PR)
The most common cause of PR is PAH, but Endocarditis, Rheumatic heart disease, Congenital conditions, and Carcinoid heart disease are causes. At least 80% of normal adults have been described to have trace to mild PR, so this amount of PR cannot be considered an abnormality unless other signs of right heart disease are found. All the RV OUTFLOW views are acceptable for assessing PR, but the apical and subcostal views should be more parallel to flow and more accurate.
The following clip shows impressive PR in the SCSAX.
Patent foramen ovale
Patent foramen ovale (PFO) occurs at the interatrial septum's peripheral, thin portion. Almost all PFOs are too small to create hemodynamically important left-to-right atrial shunts but can cause paradoxical emboli. Patients can have asymptomatic thrombi in the legs or pelvis, and small emboli may move through the right heart. These emboli often remain asymptomatic as the lungs filter and dissolve them. However, a PFO patient with the misfortune to transiently increase RA pressure through coughing or straining while a clot traverses the RA may shunt the clot to the LA, resulting in a paradoxical arterial embolus, most notoriously a TIA or stroke. An optimized SC4 view of the interatrial septum is best for seeing a PFO, but spontaneous Color across the septum is rare. Injection of agitated IV contrast during a Valsalva maneuver is the best way to detect and quantify a PFO, and the formal ultrasound lab does this when specifically requested. Here is a large PFO with a red jet moving from the left to the right atrium, acting like an atrial septal defect.
Atrial Septal Defect (ASD)
An ASD should be considered whenever a dilated, non-hypertrophied RV is seen, and meaningful TR and PR have been excluded. The largest ASDs are usually diagnosed in childhood, but hemodynamically important ASDs can be asymptomatic into adulthood. ASDs that cause RV dilation always need to be fixed.
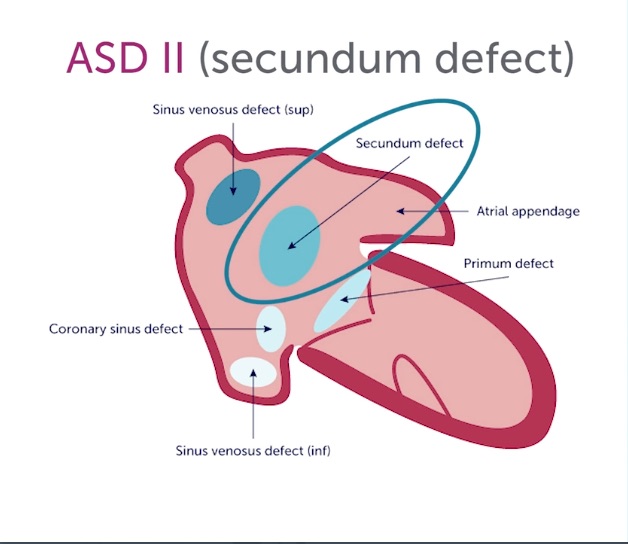
The apical4 and subcostal4 are the essential views, and Color should be applied over the interatrial septum. The subcostal4 is more sensitive for the jets because it is more parallel to flow. With high suspicion for an interatrial shunt, keep the Color sector width narrow to preserve a high frame rate. There are three types of ASD depicted in the following diagram.
Sinus venosus defects and primum ASD are rare and often require TEE to be defined. The much more common secundum ASD can be well identified with trans-thoracic echo. It occurs in the interatrial septum's thicker, more hyperechoic central part. Here is an apical4 with Color showing a smallish secundum ASD. Notice that the RA and RV were not particularly dilated, reflecting the small size of the shunt.
Here is the B-mode apical4 from a nine-week pregnant patient who was found to have RBBB on her ECG. The RV was significantly enlarged, non-hypertrophied, and had excellent function. Her mid-RVEDD was 5.4 cm.
She had mild TR with a mild TV gradient elevation, no PR, and no VSD. Here is her subcostal4 with Color showing a jet crossing the interatrial septum.
An IMBUS-detected ASD often needs confirmatory formal echocardiography, and bubble contrast may be ordered during the exam. However, the contrast study can fail to show the shunt if RA pressure is not high enough to shunt blood from right to left, even with a Valsalva maneuver. TEE, perhaps with 3D imaging, is often needed for interventional therapy planning.
With large ASDs, the PA usually dilates. Here is an angled PSAX in a patient with a large ASD and a huge PA.
Large shunts produce excess pulmonary arterial flow that can eventually result in PAH. The resulting rise in RV and RA pressures can balance the ASD shunt and make jets disappear. Fortunately, such large shunts are usually easy to see as holes in the interatrial septum with B-mode. Rarely, when PAH from a large shunt progresses far enough, the RA pressure can exceed the LA pressure, and the shunt can reverse direction, producing hypoxia. This is Eisenmenger syndrome.
CAUTION: In the subcostal4, inflow into the RA from the IVC, SVC, or even coronary sinus can be prominent and suggest an atrial shunt if the inflow Color signal appears first at the interatrial septum. The following is a good example of an inflow initially suggesting shunt to the physician.
Usually, this inflow is prominent, suggesting a good volume of shunt. Yet, the patient has no dilation of the RA or RV, so the possibility of a substantial shunt is greatly diminished. Notably, an atrial shunt should show red Color crossing the interatrial septum from the LA into the RA. The Color signal should not all be on the RA side of the interatrial septum. Left to right atrial flow should be greatest during atrial contraction with exhalation when LA pressure is highest. IVC/SVC flow into the RA is greatest during inhalation while the RA relaxes.
Ventricular Septal Defect (VSD)
Acquired VSD in ischemic disease should have accompanying LV wall motion abnormalities. Congenital VSDs are less common than ASDs. The most common congenital VSD occurs in the membranous septum near the aortic valve, so even the PSAX can see it, as in the following.
A subcostal4 is best for VSD detection because the beam is more parallel to flow through the IVS. Continuous wave Doppler should be placed through the jet because high velocity indicates a small VSD that does not need to be closed.
RIGHT VENTRICULAR DYSPLASIA (RVD)
This is an autosomal dominant genetic disease with a significant complication of arrhythmogenic sudden death, so this entity is sometimes called arrhythmogenic right ventricular dysplasia (ARVD). There is fatty and fibrous replacement of myocardium in certain parts of the RV. The ECG may have an epsilon wave in the very early ST segment. The echocardiographic features of RVD are strongly suggestive but not diagnostic. Cardiac MR is currently the best diagnostic test, showing late enhancement in characteristic areas of the RV.
The most crucial echocardiographic feature of RVD is focal dilation with accompanying reduced function and wall thinning. To make the diagnosis, the different parts of the RV must be seen, including the RVOT, which is often dilated, even in isolation. The apex may have decreased function and increased trabeculation. Because the basal RV may be spared, TAPSE and TV s’ can be normal. TR is usually present, but the gradient should not be substantially increased.
Here is an apical3R of an RVD patient showing that the lower RV is not dilated but the outflow tract going to the right is enlarged.
Here is the same patient with a SCSAX showing the dilated distal RVOT.
CARCINOID HEART DISEASE
When carcinoid tumors metastasize to the liver, vasoactive substances may be released into the hepatic veins and flow directly to the right side of the heart. These substances cause endocardial fibrosis with the most significant effect on the TV and the PV. The TV lesion is almost always TR. The PV develops PS, but PR may also occur. The endocardium of the RV wall can be thickened and more echogenic. The vasoactive substances are cleared in the lung, so the left heart is spared. Carcinoid heart disease is the primary cause of death in carcinoid syndrome.
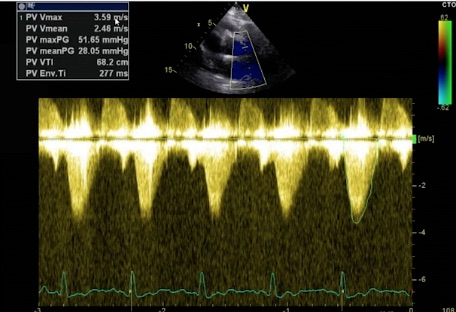
The following patient had RV dilation and moderate TR. The TV gradient measured 64 mmHg, so the initial assumption was that the patient had PAH. However, when the PV was appropriately evaluated with Color and CW, a high-velocity flow was seen through the PV. Here is the CW tracing through the PV using the SCSAX.
The PV Vmax was 52 mmHg, showing that the elevated TV gradient was not caused by PAH but by PS. Peak gradients > 36 mmHg indicate moderate stenosis, with gradients > 64 indicating severe stenosis. We could also get a dimensionless index (RVOT Vmax/PV Vmax) on such a patient as we would with suspected aortic stenosis. The details of the PV in this patient were not easy to see, but the TV was seen a little better.
The leaflets of the TV were thickened, especially the tips of the leaflets. The leaflets were restricted in movement, so they coapted poorly and caused TR. Here is a different patient with carcinoid showing thickened, restricted TV leaflets.
The combination of PS and TR is the hallmark of carcinoid heart disease. Hence, the examiner, in this case, put the probe over the liver and saw masses, and the carcinoid was eventually diagnosed. The RV does poorly with the combination of TR and PS. However, if the carcinoid tumor is treatable AND RV function is still good, valve surgery on the RV and PV is possible, and heart function and survival will improve.