Left Ventricular Failure & Left Atrial Pressure
The ever-present question of: "volume status", "fluid responsiveness", "fluid tolerant", "wet or dry"....What's the right question to ask, and how can bedside ultrasound help us determine the best treatment.
INTRODUCTION
Physicians commonly ask whether a patient is “volume up or down,” “fluid overloaded or dehydrated,” or “wet or dry.” However, in these situations, the correct clinical questions are “What is the left atrial pressure (LAP)?” and “Can the heart tolerate or improve with additional fluid?”
For decades, left heart failure (HF) was seen as the result of a disease that caused left ventricular (LV) systolic function to “fail,” and systolic function was measured as ejection fraction (EF), a measure of radial function. Many therapeutic trials were done in patients with “systolic heart failure” or what more recently is called “heart failure with reduced ejection fraction” (HFrEF). However, EF was not the whole answer.
Many patients with fatigue, exertional dyspnea, pulmonary congestion, and normal EF were identified. These patients had LVs that were normal to small in volume, “stiff,” and susceptible to elevated LV end-diastolic pressure when left-sided volume increased, or tachycardia reduced the LV's time to fill and empty. These patients often did poorly with the loss of atrial contraction during atrial fibrillation. The result was elevated LAP, causing symptoms of pulmonary congestion.
For many years, this disease was called “diastolic HF,” but more recently, the term “HF with preserved ejection fraction” (HFpEF) has been used. This condition can be seen in patients with inadequately treated hypertension or aortic stenosis, where hypertrophy of the myocardium occurs. It is also associated with aging patients, particularly women and those who are obese and diabetic, where increased fibrosis occurs in the myocardium. Hormones and cytokines from increased adipose tissue may be a key causative factor. HFpEF may also be caused by constrictive pericarditis and restrictive cardiomyopathies such as amyloid, sarcoid, Fabry disease, hemochromatosis, and endocardial fibrosis. Thus, the cause needs to be carefully considered for every patient with diastolic dysfunction. The characteristics of the US population predict that the metabolic syndrome-associated variety of HFpEF will be the dominant form of HF in primary care clinics. The BNP is often low in these patients with mild HFpEF, likely from an obesity effect, but seems to increase with more severe disease. If patients have increased LV hypertrophy because of hypertension, the hypertrophy can decrease with hypertensive treatment, but the stiffness from fibrosis is more resistant. There is also evidence that the stiffness occurs before hypertrophy in many chronic hypertension patients.
However, it is a mistake to think of HF patients as distinctly either HFrEF or HFpEF. Almost all patients with symptomatic HFrEF also have abnormal diastolic function, and most patients with HFpEF beyond the early stage have abnormal LV longitudinal systolic function. Thus, many patients with HF have a mixture of systolic and diastolic troubles.
Successful treatments for HFpEF are increasing, and adipose tissue loss may be an important part of treatment. Nevertheless, for all HF patients, the clinical findings are predominantly determined by the LAP, and optimizing this pressure with medications, including diuretics, is a prime goal of HFrEF and HFpEF treatment.
INDIRECT MARKERS:
Interventricular septal (IVS) width: Patients with ≥ moderate IVS enlargement from any cause should have diastolic dysfunction. However, non-enlarged IVS patients may still have stiff ventricles, so the lack of IVS enlargement does not exclude HFpEF.
Left atrial size: The left atrial size was previously noted to be the “hemoglobin A1c of left-sided heart disease”. An enlarged LA doesn’t distinguish between HFrEF, HFpEF, valvular disease, or chronic lone atrial fibrillation but indicates chronicity in any of these conditions. A patient may have diastolic dysfunction but a normal-sized LA because the LAP has not been chronically elevated. Symptoms might only be present when significant exertion, tachycardia, acute atrial fibrillation, or acute volume overload occur.
USING “DIASTOLOGY” MEASUREMENTS
In 2016, the American Society of Echocardiography and the European Association of Cardiovascular Imaging jointly published updated recommendations for evaluating HFpEF (J Am Soc Echocardiogr 2016;29:277-314). These groups wanted to clarify and simplify this critical assessment. Not all experts agree with everything in this consensus statement. This topic is being actively studied, and changes will come.
The best criterion to diagnose HFpEF is a pulmonary artery wedge pressure ≥ 15 mmHg at rest or ≥ 25 with standardized exercise in a patient with an EF > 50%. (Verbrugge et. al. European heart Journal 2022: https://doi.org/10.1093/eurheartj/ehab911).
Using this standard, current TDI e’ cutoffs (< 0.07 m/sec septal and < 0.10 lateral) can miss some patients. Thus, patients with symptoms compatible with HFpEF who have low normal e’ should be considered for an exercise pulmonary artery wedge pressure measurement. Some experts think that low-intensity exercise echocardiography with spectral Doppler measurements before and after can identify some patients non-invasively, but this is technically challenging.
Clinical conditions that make diastology measurements invalid or unreliable were discussed in the LV chapter but need to be emphasized. IMBUS diastology is unreliable in atrial fibrillation. Pacemaker and left bundle branch block patients also have unique problems and should be avoided. Substantial mitral annular calcification, mitral stenosis, mitral regurgitation, and aortic regurgitation directed toward the anterior mitral leaflet all confuse the measurements. Patients with mitral valve repair/replacement or basal septal wall motion abnormalities (e.g., from ischemia) should also be avoided.
QUESTION 1: IS DIASTOLIC DYSFUNCTION PRESENT?
Is the heart “stiff” and not relaxing well? For IMBUS, the answer comes from e’. The septal mitral annulus is our first location for TDI measurement. However, the lateral annulus should also be measured if the septal annulus measurement is < 0.09 m/sec or if a patient is symptomatic.
Left Ventricular Diastolic Dysfunction (LVDD): This term is used by some experts to describe asymptomatic patients with reduced diastolic function but no evidence of acute or chronic elevation of LAP. This label should not be used with aortic stenosis or untreated hypertension.
Amyloid heart disease: A practical dilemma in many LVDD patients is whether to pursue cardiac amyloidosis, an under-recognized disease (mostly TTR-amyloid). Metabolic syndrome (obesity, dyslipidemia, type 2 diabetes) will be the most common cause of LVDD. However, a series of reports identified TTR-amyloidosis in 25% of those over 80 with HFpEF, 13% of men over 60 with increased wall thickness admitted with HFpEF, 16% of TAVR patients, and even 5% of patients who had been diagnosed with hypertrophic cardiomyopathy. The approach to these patients will evolve, but when an LVDD patient does not have hypertension or features of the metabolic syndrome, we should consider amyloid because treatment for TTR amyloid has substantially improved.
QUESTION 2: IS LAP ELEVATED?
If diastolic dysfunction is present in a patient with symptoms, the term HFpEF can be used, and an estimate of current LAP gives the grade of HFpEF at that moment. Grade 1 is used for patients with normal LAP. Grade 2 is used with some evidence of elevated LAP, and Grade 3 is used for strong evidence of high LAP. The grade of HFpEF is dynamic in every patient. LA size is a marker of the chronicity and severity of LAP increase but would always take grade 2 or 3 disease to create unless atrial fibrillation was also present. Non-IMBUS physicians are familiar with the term HFpEF but not with the grading system. So, we avoid using grades and instead document “HFpEF with (no/moderate/strong) evidence of elevated left atrial pressure.” We reserve the term HFpEF for patients with at least early symptoms or with markers of elevated LAP. When writing about or discussing a patient with only LVDD, we should say, “Diastolic dysfunction is present with no evidence of elevated left atrial pressure.”
A. E/A: This is a traditional marker of LAP. The velocity of the E wave is dynamic and varies with left-sided preload, afterload, and LV contractile state. The E wave increase is the most prominent change when LAP increases; the E/A ratio rises. A patient with HFpEF can have variable E/A, depending on the LAP. If a patient’s A wave is normal, the expert consensus gave an E/A ratio cutoff of < 0.8 to label a patient Grade 1 HFpEF. An E/A ratio of 0.8 – 2.0 identifies Grade 2 HFpEF, and an E/A ratio > 2.0 characterizes Grade 3 HFpEF.
The problem with using the E/A ratio for assessing LAP is that the A wave may be reduced by left atrial dysfunction, which can accompany some etiologies of HFpEF. This causes an increase in the E/A ratio even though E may not be increased, and the patient could be misclassified. We are cautious about using E/A as a LAP marker. However, serial changes in this ratio should only be caused by LAP changes, and E/A could be part of following a patient for LAP change with treatment.
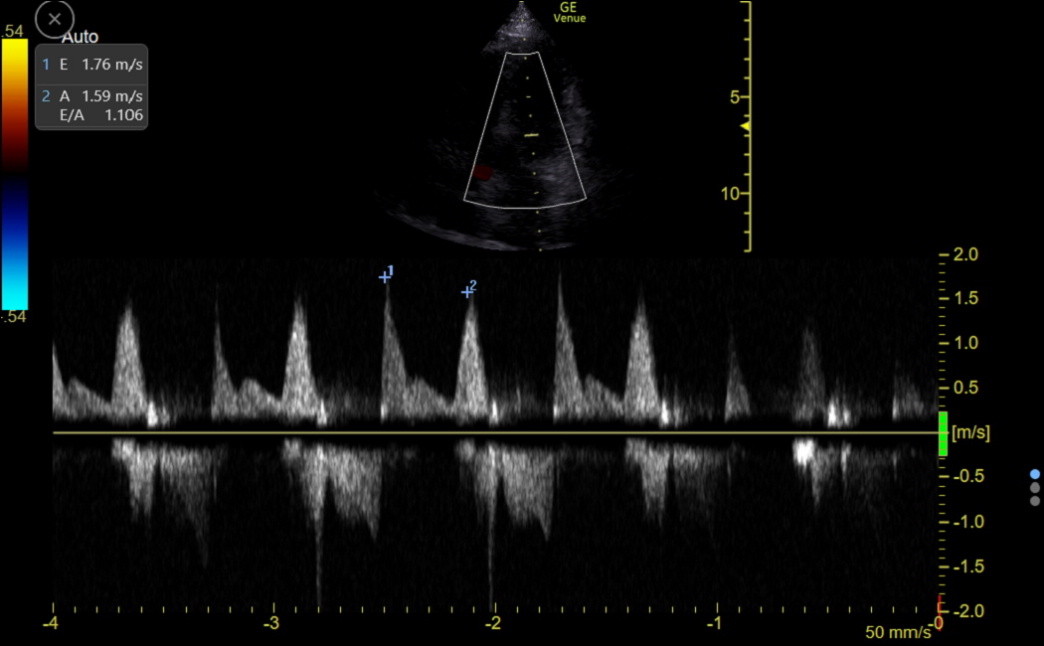
B. L wave: An L wave is uncommonly seen between the E and A waves on the LV inflow tracing. To see this detail, the sweep speed must show only three complexes on the screen. The L wave indicates that LAP is high enough to produce continued flow into the LV throughout diastole. The following clinic tracing was from an older woman with fatigue and diastolic dysfunction. The elevated E and A wave velocities and the E/A ratio of 1.1 suggested high LAP, but the L wave solidified this conclusion.
C. E/e’: Although not perfect, this is our best measure of LAP. E is produced by the push of LAP, which is higher than LV pressure at the start of diastole, and the pull of LV diastolic function. Think of E/e’ as correcting for the diastolic function pull, leaving a number that primarily reflects LAP push. The specificity of an elevated E/e’ for elevated LAP appears to be better than its sensitivity.
We are careful with the E and e’ measurements and get them at the same expiration phase, as described in the LV chapter. Using CW E and septal e’ measurements, E/e’ ≤ 10 indicates a normal or low LAP, values between 10 and 15 are indeterminate, and > 15 indicates elevated LAP. Because lateral e’ is usually higher than septal, the E/e’ upper cutoff for the lateral annulus is about 13, with a lower cutoff of about 8. Intermediate readings can indicate beginning increases in LAP but are often produced by falsely low e’ values from suboptimal gait placement. Consider all the other heart findings when interpreting intermediate E/e’.
PERICARDIAL CONSTRICTION: This uncommon cause of HFpEF has a thickened, calcified pericardium, which is often difficult to see with echocardiography. Constriction can occur because of any cause of chronic pericardial effusion and after cardiac surgery or radiation of the pericardium. The IMBUS clue to differentiating pericardial constriction from other causes of HFpEF is “annulus reversus” of e’. Constriction impairs the movement of the lateral wall of the LV more than the septum, so compared to all other situations, the lateral MV annulus e’ is lower than the septal. Thus, any patient with symptoms that could be heart failure should have both septal and lateral TDI measurements, even if the septal TDI is normal. Advanced pericardial constriction should have unusual septal motion (can be subtle). It should not have atrial enlargement, which should be present with other causes of chronic HFpEF with elevated LAP.
A FEW ADDITIONAL PRACTICAL CONSIDERATIONS
Patients with left heart failure of any type who are starting or increasing diuretic therapy should be followed to be sure they are improving but not getting too much diuresis. Serially following lung B-lines is helpful. Diastolic function does not change over short periods, so changes in the E-wave reflect the change in LAP. The E/e’ ratio (using both septal and lateral e’ for better precision) can show a patient’s progress toward a normal LAP. When there is a concern about excessive diuresis, the dynamic carotid flow time can help indicate that a patient has become fluid-responsive.
A reduced E/e’ with a fluid-responsive dynamic carotid flow time can also support a diagnosis of low LAP in a hypotensive, weak, or lightheaded patient.