Liver Region Pathology
Description and examples of the important abnormalities in the liver region that the advanced IMBUS provider should identify.
DIFFUSE LIVER DISEASE
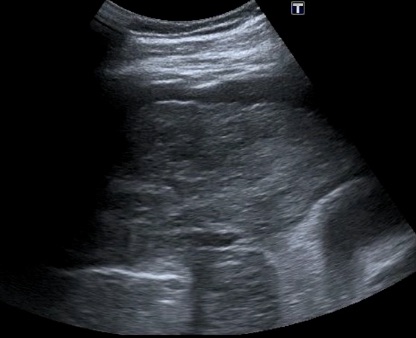
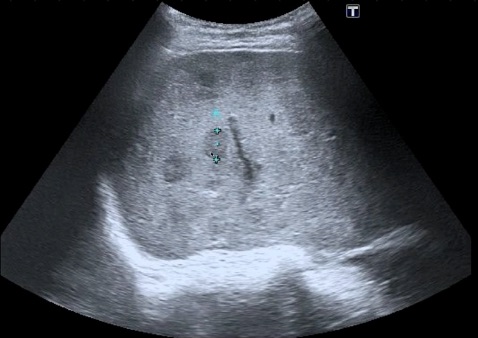
Hepatic congestion: Elevation of right heart pressure from any cause, or thrombosis of the hepatic veins (HV) (Budd-Chiari), will lead to congestion of blood in the liver. This leads to widening of the HV and hepatomegaly with a reduced echogenicity of the parenchyma. The confluence of the HV with the IVC can resemble a “bunny” as the structures enlarge. Pleural effusions and ascites may be seen in such patients. Color flow Doppler (Color) can determine whether there is flow in the HV, and regurgitant flow from tricuspid regurgitation may be seen. Chronic thrombosis in HV is usually seen as an echogenic area in a vein that obstructs flow. The following is an example of elevated right atrial pressure from tricuspid regurgitation leading to dilated IVC and HVs that resemble a bunny. The Color refluxes into the HVs with RV systole.
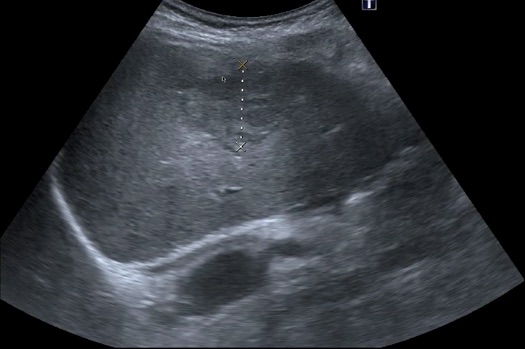
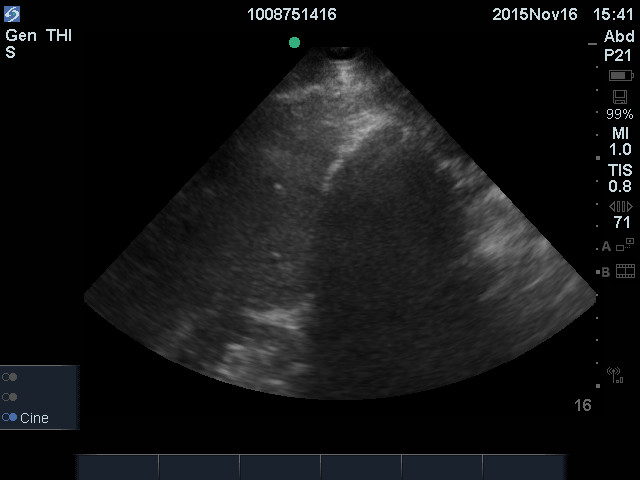
Hepatic steatosis (fatty liver): This is common and can be alcoholic or metabolic dysfunction-associated steatotic liver disease (MASLD). Chronic viral hepatitis may also show steatosis. The MASLD variety is seen in obesity, diabetes, and dyslipidemic patients. Many patients are asymptomatic, but the condition can damage the liver, and hepatomegaly is seen in more severe cases. The diagnosis of steatosis is helped by comparing the liver parenchyma to normal right kidney parenchyma. The following is an enlarged liver with MASLD in a longitudinal subcostal view.
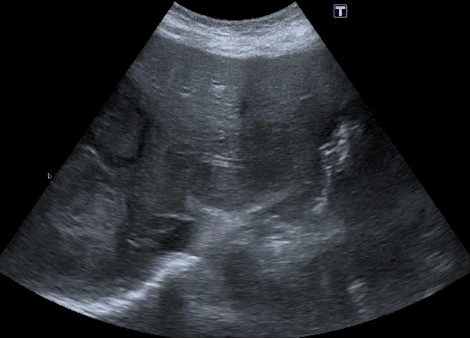
A fatty liver appears brighter than normal and more echogenic than the kidney parenchyma. Severe steatosis reduces ultrasound penetration, so the view of other liver structures may be compromised. Here is another young man with MASLD.
Liver complications from MASLD increase with the severity of hepatic fibrosis. With current treatments, hepatology referral is not needed for every patient we see with liver steatosis. We need to refer only those at increased risk for fibrosis.
Liver steatosis patients older than 50 with at least three of the following metabolic abnormalities are at increased risk of fibrosis: obesity, hypertension, hyperlipidemia, and type 2 diabetes. Type 2 diabetes is the most robust predictor of fibrosis progression, and some experts would refer all such diabetics with steatosis to hepatology.
In non-diabetic patients, liver steatosis without liver nodularity or splenomegaly can be assessed with the Fibrosis-4 Index: (age x AST)/(platelet count x ALT 1/2). A low risk of advanced fibrosis is indicated by an index < 1.3, and such patients would be followed and not referred. Online calculators for this index are easy to access:
https://www.mdcalc.com/calc/2200/fibrosis-4-fib-4-index-liver-fibrosis
A fatty liver can have focal hypoechoic areas (FHA), parts of the liver that are spared from fatty infiltration. They are most common near the gallbladder and anterior to the portal vein. The problem with these areas is that they can suggest tumors but are usually not nodular in shape, and the location helps with the diagnosis. The architecture shouldn’t change with such lesions, and hepatic and portal veins should not be displaced. Short-term follow-up with IMBUS is appropriate for equivocal cases, but MR is the definitive test to establish fat as the cause.
Unfortunately, some patients can have more alarming and very prominent FHAs, such as the following clinic patient who required several months of follow-up to show no change. Large FHAs correlate with more severe steatosis.
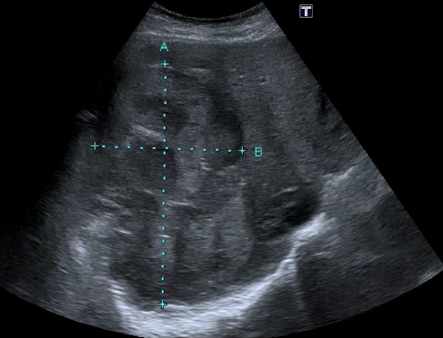
Hepatic cirrhosis: The etiology of cirrhosis can’t be determined by IMBUS findings. Advanced cases with prominent ascites and a shrunken liver with irregular borders are easy to identify. The earlier disease requires more careful attention to the liver contour, looking for subtle irregularity. The liver can be enlarged in early cirrhosis, and ascites may be absent. The liver parenchyma becomes inhomogeneous (irregular) as cirrhosis progresses, and the HVs become compressed. In contrast, the main PVs will enlarge to greater than 12 mm in diameter as portal hypertension progresses. The following image shows a small amount of ascites at the top, an irregular liver contour, and some inhomogeneity of the parenchyma.
FOCAL LIVER LESIONS
Focal, solid liver lesions are rare in our clinic. Although CT scan-detected lesions are handled differently based on size (0.5 cm cutoff) and patient malignancy risk, we are unlikely to detect solid lesions less than 0.5 cm. With our patient population, we obtain CT/MR for most solid lesions. This approach has yet to generate excess scans because the finding is rare.
-------------------------------------------------------
Hemangioma: These lesions are common, benign, often multiple, and more often seen in women because female sex hormones are associated with development. They are usually asymptomatic and incidental but can rarely cause trouble if they are large. Hemangiomas are hyperechoic with smooth borders that look like a “snowball” in the liver. They can give slight posterior acoustic enhancement because they are filled with blood vessels. Hemangiomas are occasionally less hyperechoic, and then CT/MR is usually needed to distinguish them from more dangerous lesions. Here is a typical hemangioma with subtle posterior acoustic enhancement. We likely would not get CT/MR for such a characteristic lesion.
Here is another more significant hyperechoic lesion that was confirmed to be a hemangioma.
Cysts: These are common, benign, and single or multiple. With the gain set correctly, these are anechoic, round, or oval structures with a smooth outline. They should have posterior acoustic enhancement and may have lateral shadowing (edge artifacts). It is always a good idea to use Color or power Doppler to show no blood flow in these structures. Here is a clip from a patient with multiple liver cysts, some of which were large. The cyst in this clip is entirely anechoic, with posterior acoustic enhancement and slight edge artifacts. If each cyst meets all the criteria for a simple cyst, a CT/MR is unnecessary.
Next is a patient with known polycystic kidney disease. The liver showed multiple large and small simple cysts. The dominant cyst in this clip had edge artifacts and posterior acoustic enhancement.
In contrast, the next patient had a newly seen liver cyst that had some internal hyperechoic septations that could not meet the criteria for a simple cyst. This patient needed interval follow-up and likely CT/MR.
Focal nodular hyperplasia (FNH): This is an unusual lesion seen more often in women, but female sex hormones are not associated with development. It is usually asymptomatic. The area is irregular in echogenicity and sometimes large. Color/power Doppler may show a small central artery. Unfortunately, malignancy is also a consideration with these lesions, and MR may be needed to identify FNH. Here is an ill-defined FNH lesion.
The following clip is from a 26-year-old male clinic patient. CT scan had some concern for malignancy, but MR with contrast met the criteria for FNH.
Hepatocellular carcinoma (HCC): HCC occurs mainly in patients with certain types of chronic liver disease. Even if patients are being routinely screened with formal US for HCC, they might be seen in clinic with right upper quadrant complaints in between screenings and something could be seen. The lesions can be hypoechoic or hyperechoic but are usually irregular areas. They are not as well defined as focal nodular hyperplasia. CT/MR is needed with this lesion. Here is a large and characteristically non-homogenous HCC.
This second HCC image shows two smaller lesions on the left, one more hypoechoic and the other more hyperechoic.
Metastasis: These are usually multiple, variably sized, hypoechoic, non-homogenous, and without clear margins. A hypoechoic halo is a sign of malignancy. The following image shows numerous metastases.
GALLBLADDER PATHOLOGY
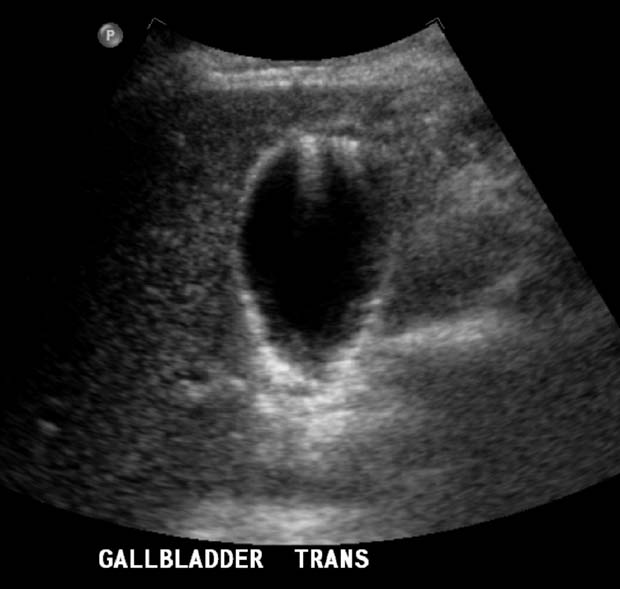
Gallbladder enlargement: Extended fasting produces the largest normal gallbladder (GB), which can be 10 cm in length and 4 cm in width for a normal-sized adult. The leading cause of a GB larger than this is distal common bile duct (CBD) obstruction, including a tumor in the head of the pancreas. Here is a GB that was enlarged by eyeball and measurement. The cause was a distal CBD cholangiocarcinoma.
Gallstones: Some can be missed by CT because they are isodense to bile. IMBUS will not miss these stones. Large gallstones are easy to see, but posterior shadowing may require adjustments to the gain. Here is an example.
Smaller gallstones, particularly those in the neck of the GB, are a concern. The acronym S-I-N (for stone in the neck) reflects the difficulty of detecting these stones and the adverse consequences of missing them. The posterior acoustic shadow can be the key finding for any gallstone, particularly in the neck. If the patient is not overly obese and you are uncertain about a shadow, decrease the gain, and the shadow may become more distinct. In general, gallstones should move with patient movement, but stones stuck in the neck may not move. Here is a gallstone in the neck of a GB that a CT scan could not differentiate from a polyp. The shadowing is noticeable with ultrasound, and this frail patient needed a cholecystostomy tube.
Other hyperechoic structures in the GB: Not every hyperechoic structure in a GB is a stone. Air can rarely get in the GB, but it rises to the top wall, with echogenic shadows coming from the top. Polyps are hyperechoic, don’t move with position change, and don’t cause shadows. Here is a small polyp defying gravity on the anterior wall and casting no shadow. Movement of the patient also didn’t move this polyp.
Structures in the GB fundus that look like polyps but have a short comet tail below them may be adenomyomatosis. This is a common degenerative/hyperplastic condition of the GB that isn’t an issue itself but seems to accompany chronic GB inflammation. The GB wall may be thickened. This condition is relatively common in older patients and occasionally raises the possibility of GB carcinoma, but the comet tails make it a benign lesion. Here is an example.
Sludge can show as hyperechoic to the bile but usually doesn't cause posterior acoustic shadows. A GB may be filled with sludge and almost isoechoic with the liver, thus resembling a mass in the inferior liver. However, the wall of the GB will still be present. What looks like sludge in the GB rarely turns out to be carcinoma, so consider all the patient's clinical features. Here is a GB that is full of sludge.
This is another patient with more subtle GB sludge. The gallbladder wall has a normal structure at the upper limits of normal. As discussed below, the middle hypoechoic layer is not pericholecystic fluid.
W-E-S Sign: The abbreviation for Wall-Echo-Shadow refers to a GB collapsing around many stones. There is a hyperechoic anterior wall, a thin hypoechoic layer representing the small residual lumen of the GB, and the hyperechoic beginning of the stones, below which is a very distinct posterior shadow. This is distinguished from a duodenum by noting that the outer layer of a duodenum is hypoechoic, and the shadow is “dirty” with B-lines coming off periodically from duodenal contents. Here is a WES sign.
Porcelain GB: This is rare and associated with carcinoma of the GB. Superficially, it can resemble a WES sign, but what is present is calcification of the GB wall. This creates a thin, calcified outer wall with a posterior shadow. There is no hypoechoic middle layer or a thicker beginning stone layer. Here is an example of a porcelain GB. Optimization of this image was probably needed to be sure it wasn’t a WES sign.
Cholecystitis: In the clinic setting, acalculous cholecystitis is rarely an issue, so the absence of gallstones or sludge makes cholecystitis extremely unlikely. If stones or sludge are present, carefully measure the thickness of the anterior GB wall interfacing the liver (short or long axis). Use an optimized GB image. As mentioned in the previous chapter, don’t measure a contracted GB wall. Normal wall thickness is less than 5 mm. There should be several layers in a thickened GB wall, and the outer layer should be hyperechoic. The hypoechoic middle layer is not pericholecystic fluid. Pericholecystic fluid is rare and would be beyond the outer wall of the GB. A thickened GB occurs in conditions other than cholecystitis (ascites, elevated right atrial pressure, and adenomyomatosis). Here is an enlarged GB wall associated with stones, sludge, and active cholecystitis.
Gallbladder tenderness (Murphy sign): Many decades ago, Dr. Murphy described placing his left index finger along the beginning of a patient’s right costal margin, extending his thumb, and abducting it into the abdomen. His experience showed him that this maneuver often placed his thumb close to the GB. He then watched for pain as the patient took a slow, deep breath.
Some have attached his name to the ultrasound exam to identify GB tenderness, but several essential things differ. First, while Dr. Murphy was guessing where the GB would be, the ultrasound exam requires that the GB fundus be located and placed at the upper center of the screen.
Second, inspiration is not necessary for the ultrasound exam. It is simpler to slowly push in on the GB with the probe to see if it is tender. If discomfort occurs, we need to know if the tenderness is specific to the GB, so we move to either side of the GB and repeat the pressure. If there is still tenderness, the tenderness is not specific to the GB.
We think Dr. Murphy would be happy to leave his name attached to the original hands-only maneuver and let us document whether the GB was tender to probe pressure.
BILE DUCT PATHOLOGY
Studies show that evaluation of the bile ducts is of little use for diagnosing patients suspected of subacute or acute cholecystitis. However, patients with more chronic symptoms could have obstruction of the common bile duct (CBD) or intrahepatic ducts, and both the proximal and distal bile ducts should be imaged.
Common bile duct: A normal distal common bile duct (CBD) is < 6 mm in diameter, but it gains 1 mm per decade in older patients and may be up to 10 mm in post-cholecystectomy patients. Normal CBDs were shown in the Liver imaging chapter. If we are imaging bile ducts near the porta hepatis with subcostal or intercostal views, the normal size is a mm or two smaller.
The following clip demonstrates multiple dilated intrahepatic ducts. This is the same patient shown above with the enlarged GB who was eventually diagnosed with a CBD cholangiocarcinoma. The CBD and intrahepatic ducts are expected to dilate in this condition. A patient with sclerosing cholangitis or a proximal cholangiocarcinoma might only have dilation of hepatic ducts with a normal-size CBD.